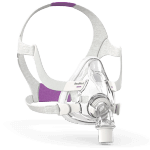
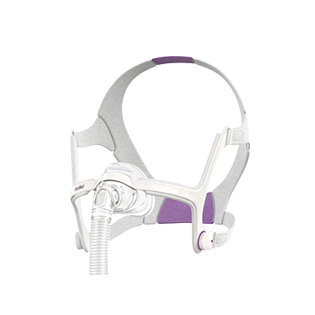
AirFit™ F20 for Her
Full-face mask
- New InfinitySeal silicone cushion designed to accommodate your movements, and tolerate misfitting
- Easy to use magnetic clips for quick fitting
- Flexible fabric-lined frame delivers breadth of fit while adding comfort
- Plush headgear assists in giving a more comfortable night’s therapy
The mask contains magnets that may interfere with certain implants or medical devices. Please refer to the user guide for complete information, including magnet contraindications and warning.
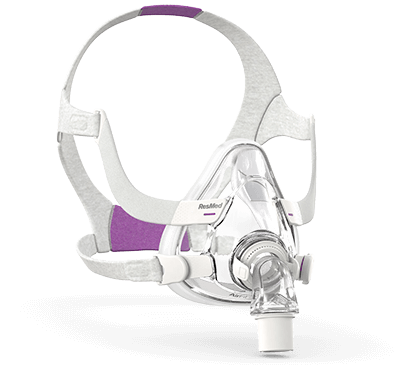

An easy fit for you
The AirFit F20 was designed to comfortably fit the widest range of faces. Indeed, in an international study, AirFit F20 fit 96.5% of all patients. 1, 2, 3, 4 The mask includes comfort features, such as the adaptive silicone cushion, which aim to provide a secure and comfortable seal for your therapy needs regardless of your face shape or size.
Patient tested and preferred
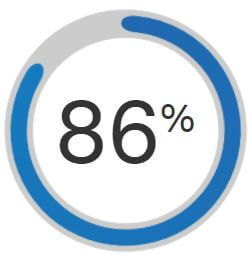
Extensively tested and designed to give you the best therapy experience, AirFit F20 is a proven choice among patients. Recent studies have shown that, when comparing against the current market-leading full-face mask, 86% of patients prefer the seal of AirFit F20.
A majority of patients also say they prefer AirFit F20 over the current market leader in terms of stability and ease of use.1 These features combine to deliver a comprehensively superior mask experience.
Our AirFit 20 for Her range
Dedicated purely to women, this range was specifically designed to give every woman a more personalised mask that better meets her unique facial features and adapts to the way she sleeps.

More products
Designed to fit every face with ease, AirFit F20 combines innovative cushion design with easy-to-use features to deliver an outstanding experience for full face mask users.
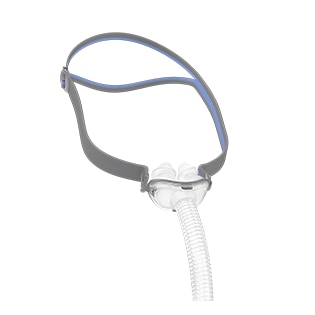
Fitting 99.4% of faces worldwide, AirFit N20’s blend of intuitive design and comfort-driven features is designed to deliver exceptional performance in a nasal mask for her.
Please refer to the user guides for relevant information related to any warnings and precautions to be considered before and during use of the products.
Contraindication
Masks with magnetic components are contraindicated for use by patients where they, or anyone in close physical contact while using the mask, have the following:
- Active medical implants that interact with magnets (i.e., pacemakers, implantable cardioverter defibrillators (ICD), neurostimulators, cerebrospinal fluid (CSF) shunts, insulin/infusion pumps)
- Metallic implants/objects containing ferromagnetic material (i.e., aneurysm clips/flow disruption devices, embolic coils, stents, valves, electrodes, implants to restore hearing or balance with implanted magnets, ocular implants, metallic splinters in the eye)
Warning
Keep the mask magnets at a safe distance of at least 6 inches (150 mm) away from implants or medical devices that may be adversely affected by magnetic interference. This warning applies to you or anyone in close physical contact with your mask. The magnets are in the frame and lower headgear clips, with a magnetic field strength of up to 400mT. When worn, they connect to secure the mask but may inadvertently detach while asleep. Implants/medical devices, including those listed within contraindications, may be adversely affected if they change function under external magnetic fields or contain ferromagnetic materials that attract/repel to magnetic fields (some metallic implants, e.g., contact lenses with metal, dental implants, metallic cranial plates, screws, burr hole covers, and bone substitute devices). Consult your physician and manufacturer of your implant / other medical device for information on the potential adverse effects of magnetic fields.
References
- ResMed internal study of 22 existing ResMed patients, conducted between 26/04/2016 – 27/05/2016 comparing the market leading mask with AirFit F20. Preliminary patient study – data on file.
- ResMed AirFit F20 internal fitting study of 27 ResMed and non-ResMed patients, conducted between 30/03/2016 – 04/04/2016. Preliminary patient study – data on file.
- ResMed AirFit F20 internal international fitting study of 34 ResMed and non-ResMed patients, conducted between 11/04/2016 – 15/04/2016. Preliminary patient study – data on file.
- ResMed AirFit F20 internal international fitting study of 90 ResMed and non-ResMed patients, conducted between 06/06/2016 – 22/06/2016. Preliminary patient study – data on file.
Content last updated: 10/2023.
 Middle-East (English)
Middle-East (English)