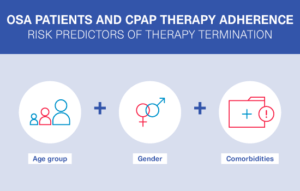
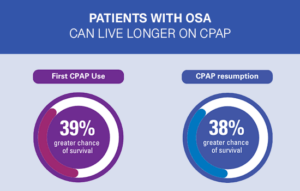
Research in sleep-disordered breathing (SDB)
Through ResMed’s strong partnerships within the global medical community, we are committed to supporting and engaging in SDB research that:
- leverages real-world evidence (RWE) to gain new perspectives on SDB patients’ responses and outcomes
- investigates the benefits of digital health technologies to support therapy adherence
- evaluates the efficacy of OSA therapies
Support for investigator initiated research
ResMed believes in the need to support ethical, independent clinical research, conducted by qualified third-party investigators.
*Système National des Données de Santé | SNDS – www.snds.gouv.fr
**Please note the Narval CC mandibular advancement device is not available in England, Scotland or Wales.
Narval CC is indicated to treat adults with snoring or mild to moderate obstructive sleep apnoea (OSA). In cases of severe OSA, it is indicated after continuous positive airway pressure (CPAP) therapy failure, non-compliance or refusal.
This content is intended for health professionals only. Please refer to the instructions for use for relevant information related to any warnings and precautions to be considered before and during use of the product.
- Pépin, J.-L.; Bailly, S.; Rinder, P.; Adler, D.; Szeftel, D.; Malhotra, A.; Cistulli, P.A.; Benjafield, A.; Lavergne, F.; Josseran, A.; et al. CPAP Therapy Termination Rates by OSA Phenotype: A French Nationwide Database Analysis. J. Clin. Med. 2021, 10, 936. https://doi.org/10.3390/jcm10050936 Retrospective analysis on 480 000 adult patients with CPAP therapy initiated from 2015 to end of 2016, identified in the French Health insurance claims database, and followed up until end of 2019
- Pépin JL, et al. Relationship between CPAP termination and all-cause mortality: a French nationwide database analysis, CHEST (2022), doi: https://doi.org/10.1016/j.chest.2022.02.013Retrospective analysis of 176 014 adult patients identified in the French health insurance claims database, who started PAP therapy between January 2015 and December 2016 and were followed up for 3 years