Symbols Glossary
An explanation of the symbols used in the product labelling of ResMed medical devices.
The following symbols do not require English text adjacent to the symbol as these are sourced from a Standards Development Organization established symbol
| Symbol | Title | Origin | Description | Notes |
|---|---|---|---|---|
 |
Catalogue Number | ISO7000: 2019-2493 | Indicates the manufacturer’s catalogue number so that the medical device can be identified. | Synonyms for “catalogue number” are “reference number” and “reorder number”. |
 |
Batch Code | ISO7000: 2019-2492 | Indicates the manufacturer’s batch code so that the batch or lot can be identified. | This symbol shall be accompanied by the manufacturer’s batch code. Synonyms for “batch code” are “lot number” and “batch number”. |
 |
Serial Number | ISO7000: 2019-2498 | Indicates the manufacturer’s serial number so that a specific medical device can be identified. | NA |
 |
Manufacturer | ISO7000: 2019-3082 | Indicates the medical device manufacturer, as defined in EU Directives 90/385/EEC, 93/42/EEC and 98/79/EC. | This symbol shall be accompanied by the name and address of the manufacturer. The date of manufacture, as well as the name and address of the manufacturer, can be combined in one symbol. |
 |
Date of Manufacture | ISO7000: 2019-2497 | Indicates the date when the medical device was manufactured. | This symbol shall be accompanied by a date to indicate the date of manufacture, expressed as in ISO 8601 as four digits for the year and, two digits for the month and two digits for the day. This symbol can be filled or unfilled. If filled, the date of manufacture as well as the name and address of the manufacture can be combined in one symbol |
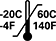 |
Temperature Limit | ISO7000: 2019-0632 | Indicates the temperature limits to which the medical device can be safely exposed. | The upper and lower limits of temperature is indicated adjacent to the upper and lower horizontal lines |
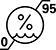 |
Humidity Limitation | ISO7000: 2019-2620 | Indicates the range of humidity to which the medical device can be safely exposed. | The upper and lower limits of humidity are indicated adjacent to the upper and lower horizontal lines. |
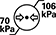 |
Atmospeheric Pressure Limitation | ISO7000: 2019-2621 | Indicates the range of atmospheric pressure to which the medical device can be safely exposed. | The upper and lower limits of atmospheric pressure are indicated adjacent to the upper and lower horizontal lines. |
 |
European Community Representative | ISO15223: 2016 | Indicates the Authorized representative in the European Community. | This symbol shall be accompanied by the name and address of the authorized representative in the European Community. |
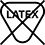 |
Latex Free |
ISO 7000:2019-2725 |
Not made with natural rubber latex | Consists of the ISO symbol “Contains or presence of natural rubber latex” with the negation symbol from IEC 80416-3 to indicate “Not Made with Natural Rubber Latex” |
 |
Contains Latex | ISO7000: 2019-2725 | Contains or presence of natural rubber latex. | NA |
 |
Contains Phthalates | ISO7000: 2019-2725 | Contains or presence of phthalates. | May include one or more additional texts identifying specific phthalates or sub-groups of phthalates, BBP, DBP |
 |
Do Not Re-use | ISO7000: 2004-1051 | Indicates a medical device that is intended for one single use only. | Synonyms for “Do not re-use” are “single use” and “use only once”. |
 |
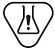
Caution | ISO7000: 2004-0434A | Indicates that caution is necessary when operating the device or control close to where the symbol is placed, or that the current situation needs operator awareness or operator action in order to avoid undesirable consequences. | The symbol variant ISO 7000-0434B (“Caution”) may be used. |
 |
Consult Instructions For Use | ISO7000: 2019-1641 | Indicates the need for the user to consult the instructions for use. | Synonym for “Consult instructions for use” is “Consult operating instructions”. |
 |
Do Not Use if Package is Damaged | ISO7000: 2019-2606 | Indicates a medical device that should not be used if the package has been damaged or opened. | NA |
 |
Use-By Date | ISO7000: 2019-2497 | Indicates the date after which the medical device is not to be used. | This symbol shall be accompanied by a date to indicate that the medical device should not be used after the end of the date shown. The date shall be expressed as in ISO 8601 as four digits for the year and, where appropriate, two digits for the month and two digits for the day. |
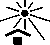 |
Keep Away From Sunlight | ISO7000: 2019-0624 | Indicates a medical device that needs protection from light sources. | This symbol can also mean “Keep away from heat”, as referenced in ISO 7000:1989. |
 |
Keep Away From Rain | ISO7000: 2019-0626 | Indicates a medical device that needs to be protected from moisture. | This symbol can also mean “Keep dry” as referenced in ISO 15223-1:2016. |
 |
Fragile, Handle with Care | ISO7000: 2019-0621 | Indicates a medical device that can be broken or damaged if not handled carefully. | NA |
 |
WEEE | EU Directive 2012/19/EU | Waste in Electrical and Electronic Equipment. | The Directive provides for the creation of collection schemes where consumers return their WEEE free of charge. |
 |
WEEE Separate Collection | EU Directive 2006/66/EC | The symbol indicating ‘separate collection’ for all batteries and accumulators. | NA |
 |
This Way Up | ISO7000: 2019-0623 | To indicate correct upright position of the transport package. | NA |
 |
General symbol for recovery/recyclable | ISO7000: 2019-1135 | To indicate that a material is part of a recovery or recycling process. | NA |
 |
Non-ionizing electromagnetic radiation | IEC60417:2002-5140 | Indicates equipment or systems e.g. medical devices, that include RF transmitters or that intentionally apply RF electromagnetic energy for diagnosis or treatment. | Also indicates generally elevated, potentially hazardous, levels of nonionizing radiation. |
 |
Type B applied part | IEC60417: 2002-5840 | On medical equipment to identify a type B applied part complying with IEC 60601-1 | B = Body. Used for applied parts that are generally not conductive and can be immediately released from the patient. May be connected to earth. |
 |
Type BF Applied Part | IEC60417: 2002-5333 | To identify a type BF applied part complying with IEC 60601-1. | BF = Body Floating, Used for devices that have conductive contact with the patient, or having medium or long term contact with the patient. May not be connected to earth (floating). |
 |
Type CF Applied Part | IEC60417: 2002-5335 | To identify a type CF applied part complying with IEC 60601-1. | Type CF, Cardial Floating, is the most stringent classification, being required for those applications where the applied part is in direct conductive contact with the heart or other applications as considered necessary. |
 |
Refer to instruction manual/booklet | ISO7010-M002 | To signify that the instruction manual/booklet must be read. | NA |
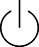 |
Stand-by | IEC60417: 2002-5009 | Indicates a sleep mode or low power state. | The switch does not fully disconnect the device from its power supply. |
 |
Direct current | IEC60417: 2002-5031 | To indicate on the rating plate that the equipment is suitable for direct current only; to identify relevant terminals. | NA |
 |
Alternating current | IEC60417: 2002-5032 | To indicate on the rating plate that the equipment is suitable for alternating current only; to identify relevant terminals. | NA |
 |
Class II Equipment | IEC60417: 2002-5172 | To identify equipment meeting the safety requirements specified for Class II equipment according to IEC 61140 | A Class II or double insulated electrical appliance is one which has been designed in such a way that it does not require a safety connection to electrical earth (ground). |
 |
Fuse | IEC60417: 2002-5016 | To identify fuse boxes or their location. | NA |
 |
Battery Check | IEC60417: 2002-5546 | To identify a control to check the condition of a primary or secondary battery or to identify the battery condition indicator. | NA |
 |
MR Unsafe | ASTM F2503-13 | An item that is known to pose hazards in all MRI environments. | NA |
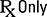 |
USA Regulatory Mark | 21 CFR 801 | Replaces “Caution: Federal law restricts this device to sale by or on the order of a physician.” | An alternative to the text “Rx Only” |
 |
IP Rating | IEC60529: 2001 | Classifies and rates the degree of protection provided against intrusion (body parts such as hands and fingers), dust, accidental contact, and water by mechanical casings and electrical enclosures. | Numbers indicate the degree of protection. |
 |
Class 9 – Miscellaneous Dangerous Goods | IATA Packing Instruction 965 Section IB | Applies to lithium ion cells with a Watt-hour rating not exceeding 20 Wh and lithium ion batteries with a Watt-hour rating not exceeding 100 Wh packed in quantities that exceed the allowance permitted in the standard. | NA |
 |
Indoor Use Only | IEC60417-2004-5957 | To identify electrical equipment designed primarily for indoor use. | NA |
 |
Alarm Inhibit | ISO60417: 2002-5319 | Indicates that the pulse oximeter monitor is not provided with a low SpO2 alarm condition. | NA |
 |
Keep out of reach of children | IEC60417:2002-6091 | Indicates devices and equipment that shall be kept out of reach of children. | NA |
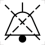 |
Bell, cancel temporary | IEC60417:2002-5576-2 | On medical ALARM SYSTEMS this graphical symbol is used as follows: To indicate that an ALARM CONDITION is in the ACKNOWLEDGED state until a time interval has elapsed. | NA |
 |
ON/OFF | IEC60417:2002-5010 | To indicate connection to or disconnection from the mains, at least for mains switches or their positions, and all those cases where safety is involved.Each position, “ON” or “OFF”, is a stable position. | NA |
 |
Universal Serial Bus (USB), Port/Plug | ISO7000:2019-3650 | To identify a port or plug as meeting the generic requirements of the Universal Serial Bus (USB). To indicate that the device is plugged into a USB port or is compatible with a USB port. | This graphical symbol is based on the USB symbol standardized by USB Implementers Forum. |
 |
Bell | IEC60417:2002-5013 | To identify switches which operate bells | To indicate an alarm system connection on a medical alarm system |
 |
Alarm, general | IEC60417:2002-5307 | To indicate an alarm on a control equipment. | NA |
 |
Importer | ISO7000: 2019-3725 | Indicates the entity importing the medical device into the locale. | If multiple symbols (i.e. Authorized Representative, Importer, Distributor, Translation, or Repackaging) identify the same responsible entity, the name and address need not be duplicated. |
 |
Contains hazardous substances | ISO 7000: 2019-3723 | Indicates a medical device that contains substances that can be carcinogenic, mutagenic, reprotoxic (CMR), or substances with endocrine disrupting properties. | The term “substances” is used to indicate a single substance or multiple substances. |
 |
Single patient multiple use | ISO 7000: 2019-3706 | Indicates a medical device that may be used multiple times (multiple procedures) on a single patient. | NA |
 |
Medical Device | N/A | Indicates the item is a medical device. | For use in Europe the full definition of “medical device” is given in EU Regulation 2017/745. |
 |
Unique device identifier | N/A | Indicates a carrier that contains unique device identifier information. | This symbol identifies the UDI carrier, including the AIDC and human readable information. |
References
- ISO 7000:2019 Graphical symbols for use on equipment – Index and synopsis.
- ISO 15223-1:2016 Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Par 1: General requirements.
- Directive 2012/19/EU of the European Parliament and of the Council of 4 July 2012 on waste electrical and electronic equipment (WEEE), Official Journal of the European Union.
- Directive 2006/66/EC of the European Parliament and of the Council of the 6 September 2006 on batteries and accumulators and repealing Directive 91/157/EEC”, Official Journal of the European Union.
- IEC 60417:2002 Graphical symbols for use on equipment.
- ISO 7010:2019 Graphical symbols – Safety colours and safety signs — Registered safety signs.
- ASTM F2503-13, Standard Practice for Marking Medical Devices and Other Items for Safety in the Magnetic Resonance Environment.
- Food and Drug Administration, 21 CFR Part 801 – Labelling.
- IEC 60529:1989/AMD1:1999+AMD2:2013 Degrees of protection provided by enclosures (IP Code).
- IATA Packing Instruction 965 Section IB, International Air Transport Association (IATA).
- ISO 15223-1:2021 – Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements.
- (EU) 2017/745, Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices. OJ L 117, Official Journal of the European Union.



