Neuromuscular disease (NMD)
While many NMDs are rare, respiratory effects are frequent,1 resulting in a substantial number of patients presenting to pulmonologists/respiratory care providers. Here, you will learn more about the respiratory effects of NMDs and about non-invasive ventilation – the key treatment modality for patients with respiratory weakness due to NMD.1
PART 1
Overview of neuromuscular diseases
What are NMDs?
Neuromuscular diseases are a heterogeneous group of neurologic conditions that directly or indirectly affect muscle function due to problems with the nerves and neuromuscular junctions.1
NMDs may be classed as neurodegenerative, autoimmune or genetic and include muscular dystrophy, motor neuron disease, peripheral neuropathy and amyotrophic lateral sclerosis, amongst others.2
Impact of NMDs on the respiratory system
NMDs are characterised by muscle weakness, which can affect the respiratory system with serious consequences.
- The incidence of respiratory effects depends upon the underlying disorder, for example respiratory failure is common in patients with amyotrophic lateral sclerosis (ALS)3
- The onset and progression of respiratory effects can be acute, gradual or acute-on-chronic, depending on the NMD1, 3
- Respiratory failure is the most common cause of morbidity and mortality in patients with rapidly progressive NMD4
The three key effects on the respiratory system are discussed next.
Decreased respiratory muscle strength
Decreased ventilatory muscle strength with a reduction in vital capacity may be present.
This is caused by a decrease in inspiratory muscle strength alone, or in combination with decreased expiratory muscle strength and increased residual volume.1
Cough function abnormalities
Cough function is vital to maintain airway clearance and prevent infection, yet impairment of cough function is common in NMD.
- This can be due to inspiratory and expiratory muscle weakness and impaired glottic function1
- In addition, sialorrhea may occur due to bulbar dysfunction1
Sleep abnormalities
Due to breathing being less efficient during sleep, sleep abnormalities are frequent and usually appear prior to diurnal hypoventilation and respiratory failure.1
Nocturnal presentations include hypoventilation and sleep-disordered breathing.
Symptoms
Symptoms of ventilatory muscle weakness (including the diaphragm and accessory muscles) can be nonspecific, occur at night (particularly for chronic NMD) and be attributed to other conditions.3, 4
- Symptoms associated with sleep-disordered breathing include: orthopnea, sleep disruption and headache upon awakening3
- Daytime symptoms of respiratory muscle weakness include hypersomnolence, worsening fatigability, increased frequency of lower respiratory tract infections, dyspnoea (positional or nonspecific),² tachypnea, and dysarthria3, 5
- Symptoms of ineffective cough include poor secretion clearance and aspiration pneumonia
Overview of neuromuscular diseases
NMDs are associated with serious respiratory system effects, which may initially present as sleep-disordered breathing prior to daytime symptoms in patients with slowly-progressing conditions. So how are respiratory effects assessed in NMD?
PART 2
NMD assessments
How are respiratory effects in NMD assessed?
The most appropriate investigations will depend on the clinical presentation. The table below summarises key investigations and the domains assessed.
- It is important that respiratory muscle weakness is monitored and detected early so treatment can be initiated in a timely manner3
- A multiple testing approach may be the most appropriate for NMDs, with repeat measurements every 3–6 months or more frequently1
- Clinical history and physical exam are required to assess bulbar weakness, weakness in diaphragm/inspiratory muscles and expiratory muscle function3
Key assessments and their findings in patients with NMD1,3,5
Pulmonary function testing
Pulmonary function testing should be carried out in the supine and upright position.3
- Measurements of respiratory muscle strength over time are useful, these include maximal inspiratory and expiratory pressure and vital capacity, including supine vital capacity which provides an indication of diaphragm weakness
- A drop in vital capacity of >19% while supine suggests diaphragm weakness; patients with bilateral diaphragm paralysis may have a drop in vital capacity of ≥50%1
- Vital capacity has been shown to be correlated with daytime and nocturnal gas exchange in NMD and is expected to decrease over time in progressive NMD6, 7
Sniff nasal inspiratory pressure
Sniff nasal inspiratory pressure can accurately predict nocturnal desaturations and respiratory failure in patients with ALS and can be useful for patients with bulbar dysfunction who may not be able to create a tight seal on the spirometer.1
Symptoms of sleep-disordered breathing
Indicators of sleep-disordered breathing include daytime hypersomnolence, nocturnal awakenings, morning headaches, decreased concentration and elevated arterial blood CO2/end-tidal PCO2/transcutaneous CO2. Sleep-disordered breathing should be confirmed with a sleep study.1
Cough peak flow
Cough function can be measured using the cough peak flow with a spirometer or peak flow meter.1
Additional assessments
Other assessments which may also be carried out include inspiratory and expiratory mouth pressures3 and chest radiograph to assess the diaphragm.

NMD assessments
A combination of assessments are required to measure respiratory function. These include clinical history and physical examination, pulmonary function testing, blood gases and sleep assessment. Next, we will discuss the available treatments for these functional impairments.
PART 3
NMD treatment
How are respiratory impairments in NMD managed?
The most appropriate management strategy depends on the underlying NMD. Respiratory failure may be temporary in NMDs that respond to therapy, while other progressive NMDs may require full-time ventilatory support.
- Supportive respiratory therapy improves survival in some NMDs such as ALS, and quality-of-life (QoL) in most patients1, 4, 6
- Regular follow-up of symptoms and sleep-disordered breathing, daytime hypoventilation and cough and swallowing effectiveness are recommended1
- Patients’ treatment goals should be discussed and will be dependent on the underlying disease and its speed of progression4
- In patients with a chronic life-limiting illness, an advanced treatment plan should be discussed early in the course of the disease4
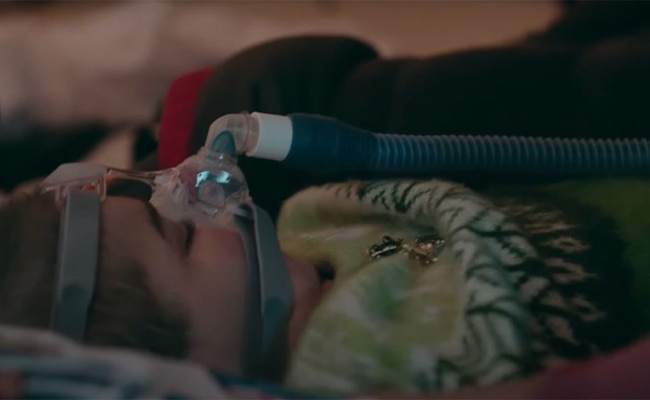
Nocturnal non-invasive ventilation (NIV)
NIV is the preferred method of ventilatory support.1, 4 NIV is associated with a correction in night and day gas exchange, increased sleep efficiency, improved symptoms and increased survival.4, 6, 7
NIV initiation
Multiple guidelines for the use of NIV exist, both in general, and specifically for NMDs;8 these guidelines vary in their recommendations for NIV initiation.
- In general, indicators for NIV initiation in NMD include sleep-disordered breathing symptoms, hypoxemia during sleep, daytime hypercapnia, decreased FVC or a rapid decline in FVC and low sniff nasal pressure3
- In contrast, bulbar symptoms are a contraindication for NIV,3 although QoL benefits have been reported in this patient population6
- Overnight monitoring is recommended following NIV initiation7 and the effectiveness of ventilation support should be monitored 1
Diurnal ventilation support
Diurnal ventilation support may eventually be required due to the progressive nature of many NMDs
- Indicators for diurnal support include elevated CO2 despite adequate nocturnal sleep therapy, diurnal dyspnoea and fatigue/difficulty concentrating1
- Diurnal support can be delivered via mask ventilation with a bi-level device, tracheostomy, or via mouthpiece ventilation to allow speech and eating1, 7
NIV modes
Ventilatory devices for nocturnal use include those with bi-level capability with a backup rate. Bi-level support modes include pressure support, pressure control, and volume-targeted modes such as average volume-assured pressure support (AVAPS) and intelligent volume-assured pressure support.1, 6
Adjusting NIV settings
The settings for NIV need to be individually adjusted for the patient:
- For bi-level support with a backup rate, pressures are usually set with an expiratory positive airway pressure starting at 4 cm H2O and an inspiratory pressure adjusted to produce a tidal volume of 6–8 mL/kg (or ~8–10 mL/kg for children)1, 7
- A backup rate of 10–12 breaths is often used and can be adjusted
- Inspiratory pressure can be increased gradually if symptoms or elevated daytime CO2 persists1
- High levels of ventilatory support (> 6.8 mL/kg predicted body weight or 9.0 cm H2O) have been correlated with lower nocturnal transcutaneous CO2 in patients with slowly progressing NMD9
NIV interfaces
Ventilation can be provided via the nose, mouth or a combination of both.6 Mask interfaces are commonly used for nocturnal NIV, these include nasal masks, oronasal masks, total face masks, and lip-seal devices.
- Choice of interface is usually according to patient and/or physician choice, as well as symptom-specific needs. Recent progress in these technologies has led to increased patient comfort and tolerance/compliance1, 7
- Mouthpiece ventilation may be preferable for daytime ventilation in patients who are able to tolerate the inferface6
Continuous positive airway pressure (CPAP)
CPAP is not recommended since hypoventilation and central events are common.1
Other supportive treatments
Supportive treatments include secretion clearance/cough augmentation, pharmacologic treatment, lung volume recruitment and respiratory muscle training.
- Secretion clearance and cough augmentation are performed via breath-stacking, manually assisted cough or mechanical insufflation-exsufflation device1, 3, 5
- Sialorrhea is treated pharmacologically with anticholinergics or botulinum toxin to decrease salivary output1
- Lung volume recruitment manoeuvres are recommended, although long-term data on the effectiveness of this intervention are lacking1, 10
- Respiratory muscle training can augment mouth pressures, improve bulbar and cough function and may help maintain the flexibility of the thoracic cage, however further supportive studies are needed to confirm long-term benefits3, 11

NMD treatment
Respiratory failure is the most common cause of morbidity and mortality in patients with rapidly progressive NMD. However supportive respiratory therapy, most commonly with NIV, has been shown to improve survival and patient QoL.12,13 Moreover, recent progress in NIV interfaces means this option is better tolerated than ever before.14
In conclusion, NIV is the foundation of respiratory therapy for patients with NMD and, together with supportive therapies, has improved the outlook in this patient population.
This content is intended for health professionals only.
References
- Benditt, J. O. Respir Care 2019, 64 (6), 679-688. DOI: 10.4187/respcare.06827.
- Mayo Clinic. Neuromuscular disease overview. 2022. https://www.mayoclinic.org/departments-centers/neuromuscular-disease-group/overview/ovc-20443670 . (accessed March 2023).
- Pfeffer, G.; Povitz, M. Degener Neurol Neuromuscul Dis 2016, 6, 111-118. DOI: 10.2147/DNND.S87323.
- Ambrosino, N.; Carpene, N. et al. Eur Respir J 2009, 34 (2), 444-451. DOI: 10.1183/09031936.00182208.
- Mary, P.; Servais, L. et al. Orthop Traumatol Surg Res 2018, 104 (1S), S89-S95. DOI: 10.1016/j.otsr.2017.04.019.
- Carmona, H.; Graustein, A. D. et al. Annu Rev Med 2023, 74, 443-455. DOI: 10.1146/annurev-med-043021-013620.
- Fauroux, B.; Khirani, S. et al. Front Pediatr 2020, 8, 482. DOI: 10.3389/fped.2020.00482.
- Wang Z, W. M., Dobler CC et al. Noninvasive Positive Pressure Ventilation in the Home. Table G.3, Guidelines for Neuromuscular Disease. rockville (MD): Agency for Healthcare Research and Quality (US), 2019. https://www.ncbi.nlm.nih.gov/books/NBK554178/table/appg.tab3/ (accessed March 2023).
- Leotard, A.; Delorme, M. et al. Sleep Breath 2022. DOI: 10.1007/s11325-022-02658-3.
- Sheers, N.; Howard, M. E. et al. Respirology 2019, 24 (6), 512-520. DOI: 10.1111/resp.13431.
- Bausek N, B. T., Aldarondo S. The power and potential of respiratory muscle training. In Respiratory equipment and devices exhibition magazine, 2018. https://www.pnmedical.com/wp-content/uploads/2018/02/White-Paper-The-Power-and-Potential-of-Respiratory-Muscle-Training-v2.pdf
- Kleopa KA, Sherman M, Neal B, Romano GJ,Heiman-Patterson T. Bipap improves survival and rate of pulmonary function decline in patients with ALS. J Neurol Sci 1999; 164: 82–88. DOI: 10.1016/s0022-510x(99)00045-3.
- Lyall RA, Donaldson N, Fleming T, et al. A prospective study of quality of life in ALS patientstreated with noninvasive ventilation. Neurology 2001;57: 153–156. DOI: 10.1212/wnl.57.1.153
- Nava S, Navalesi P, Gregoretti C. Interfaces and humidification for noninvasive mechanical ventilation. Respir Care. 2009;54(1):71-84. https://pubmed.ncbi.nlm.nih.gov/19111108/